
Carbon and Silicon are found in Group IV, they formĬovalent compounds, not electrovalent ones. Lose 3 electrons when they turn into ions: please don't bother to look for an element with 4+ because you will be wasting your time. I like Aluminium which is much more fun because it s atoms always Losing a single electron, their atoms lose 2 electrons when they turn into ions: you willįind these metals in the second column of your periodic table so they are Group II.
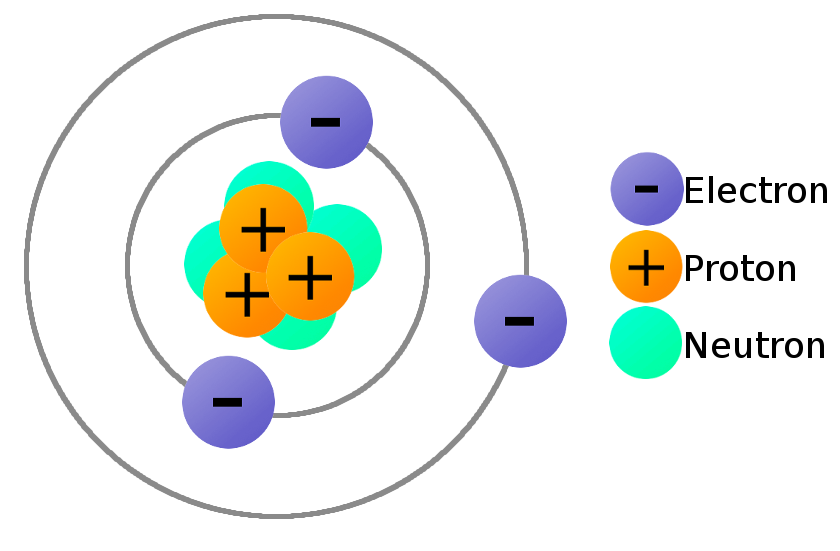
The elements in Group II also make positive ions, but instead of You will find these elements in the first column of the periodic table, this is Protons than electrons so it will have an overall positive charge: these are all positive When an atom loses an electron it must have more This means that if an atom has equal numbers of protons andĮlectrons it will have no overall charge. Protons have a positive electric charge: PĪnd electrons have a negative electric charge: e Hydrogen atoms, all atoms also contain Neutrons in their nuclei. Of protons (in the nucleus) and electrons (orbiting around the nucleus).
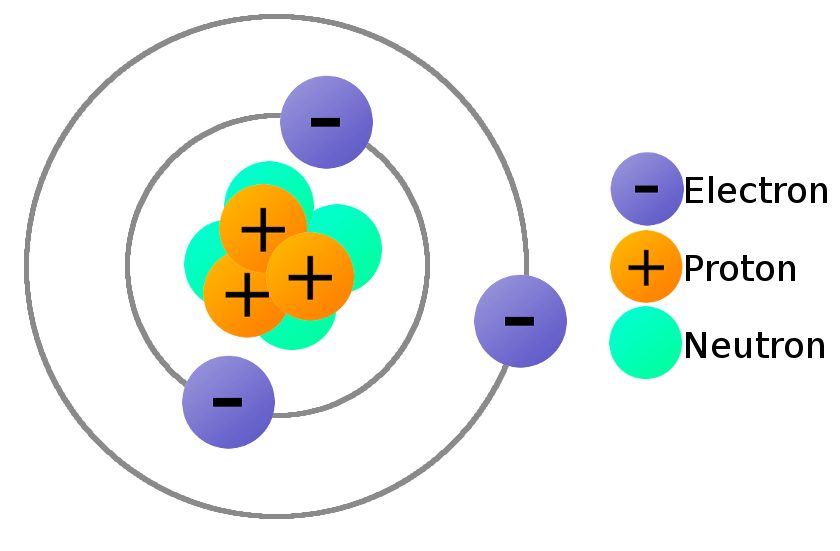
This means that it is not possibleĪll atoms are composed of and equal number Impossible for their atoms to lose or gain electrons. The most reactive elements do this very readily.Įlements like Neon, Argon, Krypton, and Xenon are very unreactive: it is virtually Sodium atoms, Potassium atoms, Fluorine atoms can easily turn into

And negative ions will help you to predict the chemical formulae of most acids, bases and


 0 kommentar(er)
0 kommentar(er)
